Research Summary
India's healthcare transformation under Atma Nirbhar Bharat and the BioE3 (Biotechnology for Economy, Environment and Employment) vision demands science that is mechanistically deep, translationally actionable, and nationally scalable.
Aligned with the mandate of BRIC-RGCB to advance translational biotechnology, precision medicine, and innovation-driven healthcare solutions for national impact, our laboratory operates at the interface of mechanobiology and therapeutic innovation. In synergy with India's Atma Nirbhar Bharat vision and the BioE3 (Biotechnology for Economy, Environment and Employment) mission, we focus on converting fundamental disease biology science that is mechanistically deep, translationally actionable providing nationally scalable, affordable, and globally competitive healthcare solutions.
We investigate how pathological mechanical forces drive the onset and progression of major diseases-including cancers, diabetes, stroke, cardiovascular disorders, neurodegeneration, and accelerated aging-conditions that impose substantial societal and economic burden. Beyond classical solid (ECM stiffening) and fluid (hydrostatic and shear) forces, we decode unconventional force-generating agents such as hyperosmotic stress from high glucose, macropinocytosis-induced intracellular fluid pressure, extracellular acidosis, and secreted galectins and amyloids. These factors regulate membrane phase separation, tensional homeostasis, and mechanotransductive signaling, shaping disease progression and therapeutic resistance.
Positioned within the emerging domain of mechanotherapy, our mission is to identify mechanosensors, adaptive effectors, and stress-responsive molecular circuits that serve as diagnostic, prognostic, predictive, and therapeutic targets. Through integrated multi-omics, functional disease modeling, and validation using indigenous bioactive resources, we develop biomarker-guided, low-toxicity mechanotherapeutics.
Our translational framework advances two strategic themes:
- Cancer mechanobiology and precision mechanotherapeutics - disrupting tumor stress adaptation and recurrence pathways.
- Cerebrovascular-cardiovascular and aging biology - restoring mechanical and metabolic homeostasis to enhance regeneration and tissue resilience.
By transforming mechanobiological insights into intellectual property, diagnostics, and affordable therapeutic leads, our work strengthens India's biotechnology ecosystem, reduces import dependency, and delivers innovation-driven healthcare solutions with both national relevance and global impact.
Research Programs
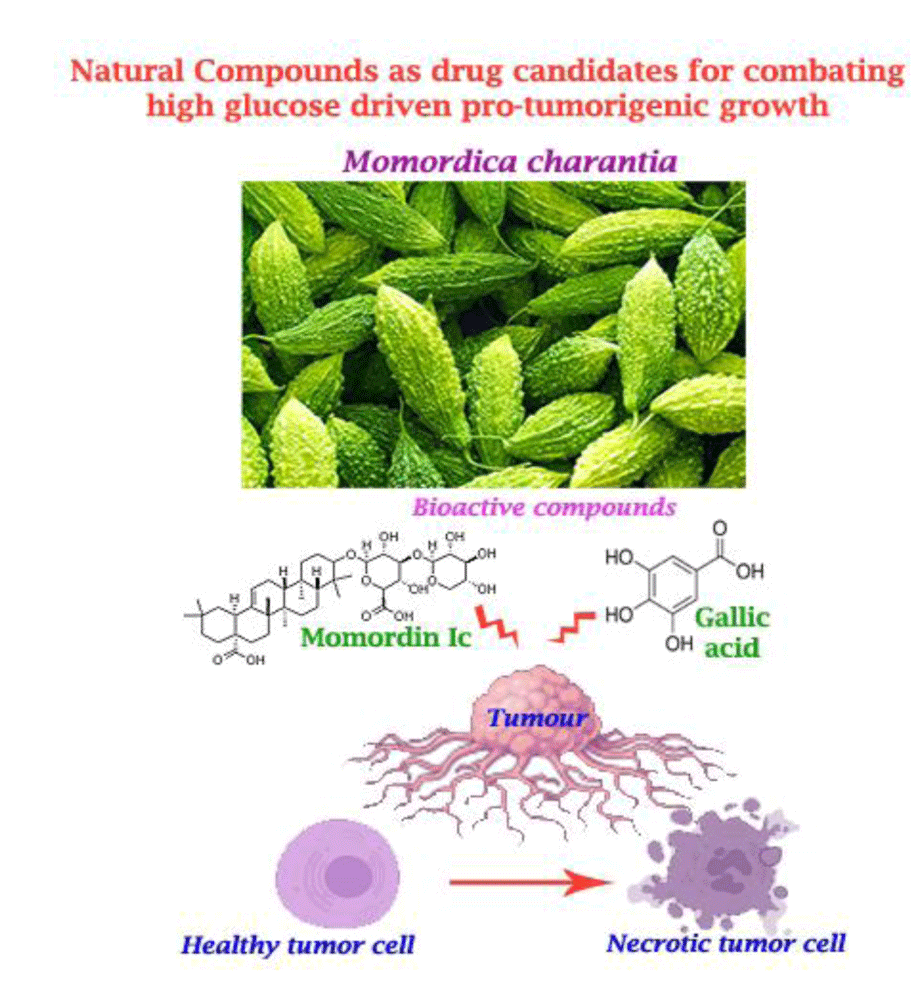
- Mimicking the biomechanical and biochemical microenvironment of primary and recurrent cancers (of brain, breast cancers, oral cavity, cervix, prostrate) for identification of the novel clinically relevant non-invasive diagnostic, prognostic biomarkers and therapeutics.
- Targeting the SUMOylation and glycosylation pathways for novel therapeutic intervention, particularly for overcoming chemotherapy resistance, metastasis, and immune evasion.
- Novel in silico and physico-chemical approaches in identification of the diagnosis, prognostics and therapeutics of cancers, as well as, development of microfluidics based cost effective drug testing platforms.
- Targeting Extracellular Matrix Stiffness and remodelled plasma membrane in Glioblastoma multiforme and invasive ductal carcinoma for inhibiting self renewability/recurrence, drug resistance and for to enhancing proficient immune cell infiltration.
- Mechanotype Profiling of circulating tumour cells and vesicles to predict chemotherapy sensitivity

Characterization and targeting of the pressure overload and high glucose hyper-osmotic stress driven diabetic biomechanical signalling in left ventricular hypertrophy leading to heart failure.

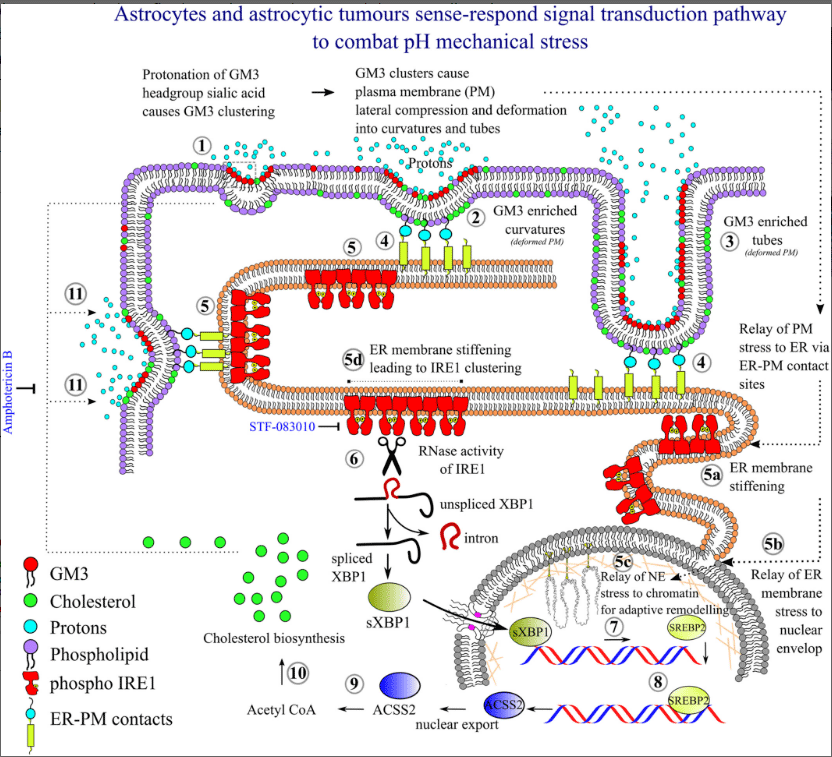
- Understading the biomechanics of glial cells in normal vs abnormal vascularisation in hybrid brain organoid and animal model: Implication on brain health and cognition.
- How neurons and neural stem cells sense and respond to the biomechanical stresses: Implication on brain regeneration therapeutics.

Current Research Grants
-
ASPIRE Grant In Inter Trans-disciplinary sciences
Council of Scientific and Industrial Research (CSIR) -
Stem Cell and Regeneration Biology Taskforce grant
Department of Biotechnology; Govt. of India. -
Therapeutics for Pressure Overload Mediated Cardiac Hypertrophy and Heart Failure, Biomedical Division Taskforce grant
Indian Council of Medical Research(ICMR); Govt. of India.
Previous/ Completed Research Grants
-
-
Ramalingaswami Fellowship Award , BT/RLF/Re-entry/16/2011;
Department of Biotechnology; Govt. of India -
Rapid grant for young investigator award (RGYI); BT/PR6331/GBD/27/403/2012;
Department of Biotechnology; Govt. of India -
Neurotaskforce grant; BT/PR4959/MED/30/770/2012;
Department of Biotechnology; Govt. of India.
-