Research Summary
The main focus of our lab is to understand the early developmental cues that promote neural stem cell maintenance and fate specific differentiation which will shed light in developing possible therapeutic strategies against neurodegenerative diseases. Our research programs are very clearly classified into two broad programs: 1) To understand the basic cues that are responsible for the various functions such as maintenance of neural stem cells, fate specification, differentiation and how the differentiated cells form proper connections in various regions of developing brain. We study these fundamental mechanisms in the developing brain and retina by modulating various extrinsic and intrinsic components such as Notch and Wnt signaling, transcription factors and miRNAs in neural cell lines and transgenic/Knockout mouse models. 2) The second part focuses on mimicking the early developmental cues to enhance proliferation of endogenous stem cell population, fate specific differentiation and formation of proper synaptic connections. We also use ES cells, Umbilical cord blood derived mesenchymal stem cells and disease modeled iPS cells for generating fate specified neurons/organoids and understanding its therapeutic potential and pathophysiology. To understand the above we mainly depend on transgenic/conditional knock out mouse models, next generation sequencing, electrophysiology and behavioral experiments.
Research Programs
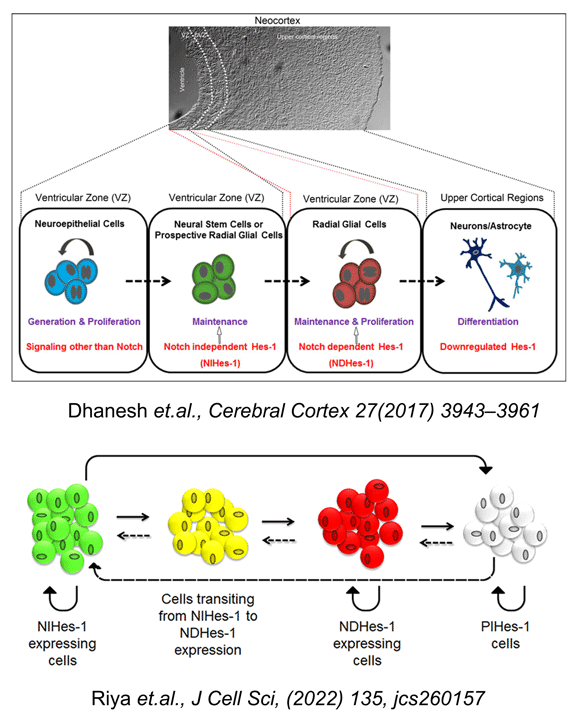
During the development of mammalian nervous system, neurons and glia are generated from a common germinal zone containing both neural stem cells (NSCs) and neural progenitors (NPs). The regulatory events that distinguish between these two proliferative neural cell types and their fate, remain poorly understood. We have previously shown the presence of neural stem cell/progenitor population which are maintained by FGF2 through activation of Hes-1, a well-established Notch receptor target. Surprisingly this activation of Hes-1 in these cells was independent of Notch and was mediated non-canonically through JNK-ATF2 signaling (Sanalkumar et.al. J. Neurochem., 113 (2010) 807-818; Sanalkumar et al Cell. Mol. Life. Sci., 67 (2010) 2957-2968). Further extrapolating our results in vivo using in utero electroporation with reporter systems, we have demonstrated that in developing neo-cortex, Notch-independent Hes-1 (NIHes-1) expression is restricted to neural stem cells and Notch-dependent Hes-1 (NDHes-1) expression is found in neural progenitors/radial glial cells (Dhanesh et al, Cerebral Cortex; 2016, 1-19, DOI: 10.1093/cercor/bhw207). This basically gave an insight to the significant differences between two very closely related populations. Though Hes-1 expression is maintained in neural progenitors, a transition from Notch-independent to dependent state makes it pleotropic as the former maintains the neural stem cells in a non-dividing/slow dividing state, whereas the latter is very much required for maintenance and proliferation of radial glial cells.
In addition to Notch signaling in neocortical development, we looked into another signaling mediated by Wnts since wnt signaling has shown significant roles in CNS development. We observed that Wnt5a, in a non-canonical manner, regulates cerebellar development which is not clearly understood. The development of cerebellum with well-defined cytoarchitecture involves multiple regulatory mechanisms during embryonic and postnatal development. In this study, we have unraveled the spatiotemporal expression pattern of Wnt5a along with its functional significance in cerebellum. Using Nestin-Cre mediated Wnt5a conditional knockout model, we demonstrated that Wnt5a mediates the proliferation of cerebellar GABAergic and glutamatergic neural progenitors in a dynamic manner which consequently affects the differentiation of respective neurons (GABAergic and glutamatergic) during early and late phase of development. In addition, we showed that Wnt5a exerts its function in a non-canonical manner and at molecular level regulates the expression of genes associated with proliferation such as cyclin D1 and Sox2 which further is in line with our in vivo results. These findings prove Wnt5a signaling as one of the crucial regulator of cerebellar development. Further investigation to identify the pathway through which Wnt5a regulates proliferation may provide useful insights in better understanding of cerebellar disease pathogenesis that are caused due to deregulation of Wnt signaling (Subashini et.al., Sci. Rep: 2017, 7, 42523; doi: 10.1038/srep42523).
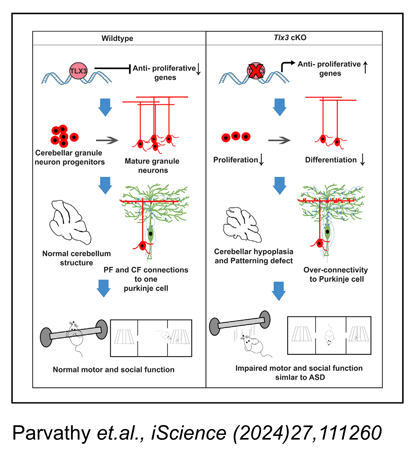
Neurogenesis is a developmental process by which new neurons are generated from neural stem cells whereas neuronal subtypes are generated through fate specification from the progenitor pool. Neuronal subtypes mainly include excitatory and inhibitory neurons which utilize glutamate and GABA neurotransmitters, respectively. Different factors regulate neurogenesis and neuronal subtype specification in a highly sophisticated manner. A set of genes known as terminal selector genes are capable of inducing or specifying a particular neuronal fate over its complementary fate. One such important gene is Tlx3 that belongs to the Tlx family of Homeobox domain transcription factors, which has been identified as a major selector gene that determines the glutamatergic neuronal fate over GABAergic fate in the embryonic spinal cord. Therefore, it would be ideal to understand the factors that regulate Tlx3 expression and also the functional significance of the progenitors/neurons that express Tlx3. We found that Hes-1 can play a critical role in deciding the excitatory versus inhibitory fate of neural progenitors by regulating the expression of Tlx3 gene which is involved in promoting glutamatergic differentiation (Indulekha et al., Cell. Mol. Life. Sci, (2012) 69:611-627). It is known that Tlx3 is expressed in post-mitotic neurons of CNS and is known to promote glutamatergic differentiation. Contrary to this, we discovered that Tlx3 is expressed in the proliferating progenitors of the external granular layer in the cerebellum, and examined factors that regulate this expression. Using Pax6-/-Sey mouse model and molecular interaction studies we demonstrated that Pax6 is a key activator of Tlx3 specifically in cerebellum, and induces its expression starting at embryonic day (E)15. By Postnatal day (PN), Tlx3 was found to be expressed in a highly restricted manner in the cerebellar granular neurons of the posterior cerebellar lobes, where it is required for the restricted expression of nicotinic cholinergic receptor-α3 subunit (Chrnα3) and other genes involved in formation of synaptic connections and neuronal migration (Divya et al, Sci Rep, (2016) 30337, DOI: 10.1038/srep30337). To further confirm our findings regarding Tlx3/Chrnα3 cascade, we analyzed Chrnα3 expression and found that its expression was entirely absent in Pax6-/- Sey cerebellum. Also we observed that Chrnα3 was co-expressed with Tlx3 in the post-mitotic EGL and in the cells that are migrating into the prospective IGL. Since Chrnα3 is linked with neurodevelopmental disorders such as Autistic Spectral Disorders (ASD), we are currently looking into the possibility whether Tlx3 could play a role in ASD.
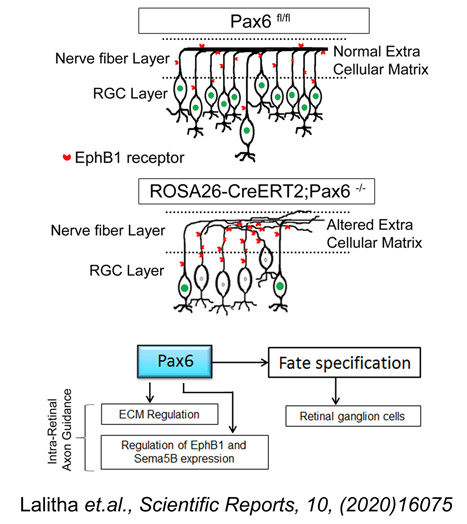
Glaucoma is the second leading cause of blindness in the world. It is a chronic disorder caused due to the progressive loss of retinal ganglion cells (RGCs) and their axons which transmit the visual information from the eye to the brain. Elevated intra ocular pressure (IOP) remains the etiological factor towards which major therapeutic efforts are directed, although none of the treatment modalities could bring back the lost RGCs and hence the visual function cannot be restored. We are currently looking into a possible method for increasing the number of RGC by transplanting ES cells that has been induced to differentiate into RGCs. Such a strategy will help in replenishing the lost RGCs to some extent. Towards this end our laboratory has successfully optimized a novel protocol for generating RGCs from ES cells by modulating the extrinsic growth factors and manipulating the neurogenesis signaling pathway (Jagatha et al. BBRC; 2009; 230-235). These ES cell-derived RGCs were able to integrate into the host retina and were proven to be functional when transplanted in glaucoma animal models through better cell integration and vision recovery compared to that of the non-transplanted animals. Our results have shown that partial visual functions can be reversed in RGG depleted NMDA model by transplantation of ES cell-derived RGCs. Success of the above strategy could help to treat glaucoma using cell replacement therapy. As part of a parallel program to identify heterologous sources for neural/RGC differentiation we have identified a very unique population of progenitors from umbilical cord blood-derived mesenchymal stem cells which has an inherent potential to differentiate into neurons (Divya et.al. Stem Cell Research & Therapy, 2012; 3:57). The remaining MSCs have to be transdifferentiated into neurons with combination of growth factor exposure for a longer time period. In addition to addressing the above problems, our lab also concentrates on identifying the miRNAs and their role in RGC differentiation and axonal extension to the visual centers of the brain. In view of the recent advances in post-transcriptional regulation by miRNA in retina we have looked into the miRNAs that could potentially target RGC specific Marker, Brn3b and thereby regulate RGC differentiation during development. We adopted an in silico approach to predict the miRNAs involved in regulating Brn3b and selected mmu-miR-23a and mmu-miR-374 for further analysis, since these two were picked up by multiple programs. We were able to demonstrate for the first time, that these two miRNAs, not individually but in combination, would affect the expression of Brn3b in the ganglion cell layer of retinal explant cultures. Also, we provide evidences for the existence of a co-ordinated mechanism controlling the wave of Brn3b expression mediated through miR-23a and miR-374, which will ultimately regulate the development of RGCs from their progenitors and later their survival and axonal guidance (Rasheed et al 2014, Developmental Neurobiology, 74: 1155-1171). Although we have observed an integration of ES-NPs in the adult retina and expressing RGC markers, the details regarding the functional attributes such as axonal extension and guidance are lacking. Hence, currently we are focusing on the molecules involved in the axonal extension process and their regulation using cKO mice models.
Current Research Grants
-
2025 2022
Functional relevance of a unique subclass of Notch independent Hes-1 (NIHes-1) expressing neural stem cells in developing/adult cortex
Science and Engineering Research Board [DST-SERB]
Previous/ Completed Research Grants
-
-
Development of a low-cost Anosmia screening tool to mass screen asymptomatic COVID-19 carriers
Science and Engineering Research Board [DST-SERB] 2020-2021 -
Regulation of stemness by Pleiotropic Hes-1 expression in neuroblastoma
National Bioscience Career Award, Dept. of Biotechnology, Govt. of India 2018-2021 -
Expression of Notch independent Hes-1 (NIHes-1) specifically in ES derived organoids representing developing neocortex: Understanding its functional significance.
Department of Biotechnology [DBT] 2018-2020 -
Guiding retinal ganglion cell axons to brain visual centers: Is Pax6 the key molecule?
Department of Science & Technology [DST] 2017-2020 -
Transcriptional regulation of Tlx3 by Pax6 and its role on excitatory neural fate specification in vitro and in vivo.
Kerala State Council for Science, Technology and Environment [KSCSTE] 2014-2016 -
Characterization of Notch independent Hes-1 mediated maintenance and fate specification of neural progenitors.
Department of Biotechnology [DBT] 2013-2016 -
Transcriptional regulation of Tlx3 (Hox11L2) by Notch signaling and its involvement in excitatory vs. inhibitory fate specification of neural progenitors.
Department of Science & Technology [DST] 2013-2016 -
Differentiation of UCB stem cells into lineage specific cell types for therapeutic applications.
Department of Biotechnology [DBT] 2008-2011 -
Isolation and characterization of human embryonic stem (ES) cell line.
Kerala State Council for Science, Technology and Environment [KSCSTE] 2007-2009 -
Development of a cell therapy strategy to treat glaucoma using retinal ganglion cells (RGCs) generated from embryonic stem (ES) cells.
Department of Biotechnology [DBT] 2006-2009 -
Transplantation of embryonic stem (ES) cells engineered to produce GABA into hippocampus of rats with temporal lobe epilepsy for possible control of seizures.
Department of Science & Technology [DST] 2004-2008
-